Potassium Hydroxide and Sulphuric Acid
It is less potent than potassium permanganate. Water Softener Salt.

Write The Net Ionic Equation For The Reaction Between Sulfuric Acid And Potassium Hydroxide Homework Study Com
Oxalic acid g Milk of magnesia.

. When an acidic medium is provided potassium permanganate can gain five electrons to make a manganese oxide ion. Liquid chlorine Sodium Hypochlorite 125. Sodium hydroxide f Window cleaner.
Ammonium hydroxide h Curd. If the potassium manganateVII solution is made slightly alkaline often by adding sodium carbonate solution the purple solution first becomes dark green and then produces a dark brown precipitate. Acetic acid i Ants sting.
It was also known as vitriolic tartar and Glasers salt or sal polychrestum Glaseri after the pharmaceutical chemist. The chemical formula of this crystallised ionic solid is K 2 Cr 2 O 7. Lactic acid e Tamarind.
4032 1985 2 Continued from page 1Members Representing SHRI A. Potassium Hydroxide Flakes Liquid Flocculent. GOKAK Development Commissioner for Cement Industry Ministry of Industry New Delhi SHRI S.
Water Softener Resin. Hydrochloric Acid 28 Solution. Water Softener Anion Resin.
Calcium hydroxide b Oranges. The only solvents which will attack PEEK are concentrated nitric acid and sulphuric acids. GUPTA Hyderabad Industries Limited Ballabagarh SHRI.
The reactions are done in the presence of dilute sulphuric acid. Welding Torch Coolant. However PEEK tubing can safely withstand the 20-30 nitric acid used for system passivation.
Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed Expired - Lifetime Application number US178960A Inventor David C Gattiker Gerhard J Frohlich Current Assignee. Most commonly the acidic medium is provided by sulphuric acid from which the sulfate ion of manganese sulfate is obtained. The name of the salt is made by taking the first.
Formic acid c Vinegar. Chemistry of the reaction. Trisodium Phosphate Tech Grade Sulphuric Acid 98.
Hydrogen Peroxide 50 Food Grade Hydrochloric Acid 33 Solution. Hydrogen peroxide and potassium permanganate undergo a redox reaction in an acidic medium. Citric acid d Lime water.
MIGLANI Alternate SHRI S. Produced by adding acid to potassium chromate it is soluble in water and insoluble in alcohol. The highest temperature we recommend for PEEK is 100C.
If the potassium manganateVII solution is acidified with dilute sulphuric acid the purple solution becomes colourless. Nitric acid acidulation acid rock phosphoric acid Prior art date 1962-03-12 Legal status The legal status is an assumption and is not a legal conclusion. It is a hexavalent compound meaning the chromium in it.
Methylene chloride DMSO and THF may cause swelling in PEEK. In either case you would pipette a known volume of solution containing the ironII ions into a flask and add a roughly equal volume of dilute sulphuric acid. GOPINATH The India Cements Limited Madras SHRI T.
Potassium Dichromate is an oxidising agent used for industrial applications. Up to that temperature the tubing will maintain the stated. Potassium sulfate K 2 SO 4 has been known since early in the 14th centuryIt was studied by Glauber Boyle and TacheniusIn the 17th century it was named arcanuni or sal duplicatum as it was a combination of an acid salt with an alkaline salt.
When an acid alkali neutralisation reaction happens - like when adding sodium hydroxide to sulfuric acid - it produces sodium sulfate and water. Sulphuric Acid 2 S M S Sulphuric Acid 50 S U U Sulphuric Acid 96 S U U Tannic Acid 2 S S Tartaric Acid S S S Tea sol S S S Tetrahydrofuran S S S Tetralin M S S Thionyl Chloride U Toluene M S M Trichloroethylene 111 U S S Softens Nylon Trisodium Phosphate S S M Triethylene Glycol. What happens next depends on whether you are using potassium manganateVII solution or potassium dichromateVI solution.
Magnesium hydroxide j Spinach. Tartaric acid a Soap. TAMILAKARAN Alternate SHRI A.

Balance Koh H2so4 K2so4 H2o Potassium Hydroxide And Sulfuric Acid Youtube

Balance Koh H2so4 K2so4 H2o Potassium Hydroxide And Sulfuric Acid Youtube
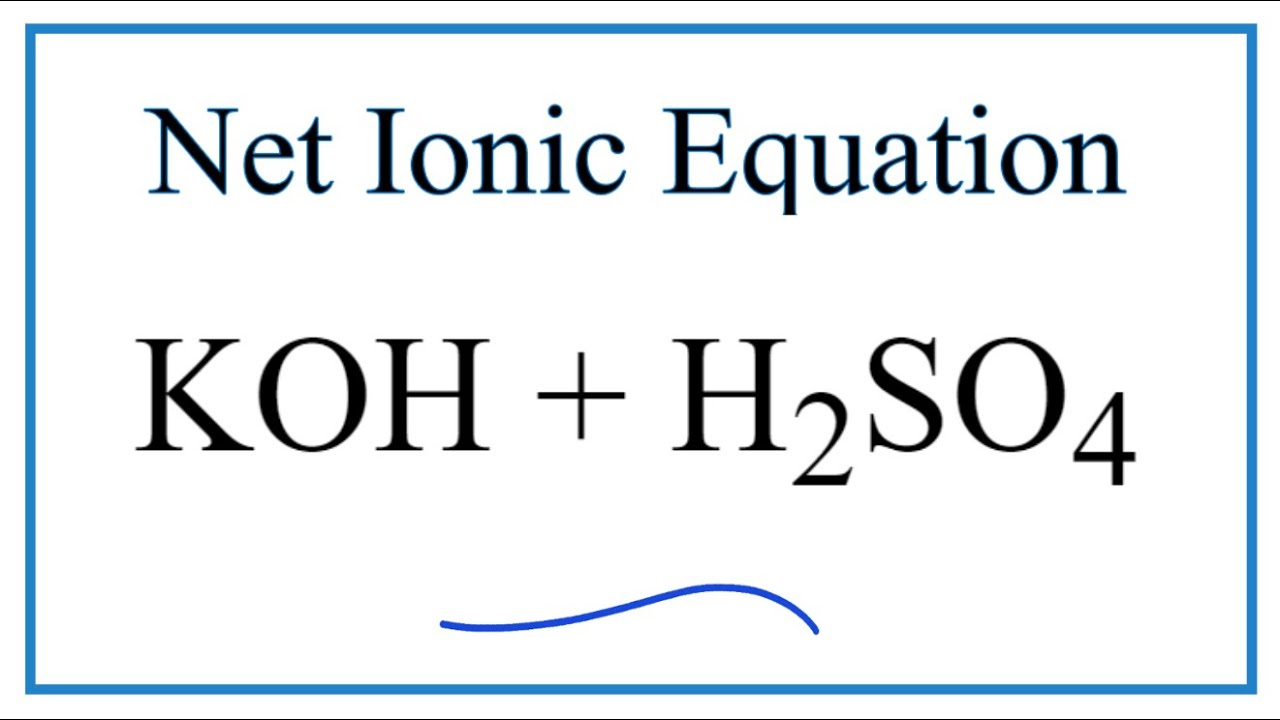
How To Write The Net Ionic Equation For Koh H2so4 K2so4 H2o Youtube

0 Response to "Potassium Hydroxide and Sulphuric Acid"
Post a Comment